Airglow is the natural “glowing” of the Earth’s atmosphere. It happens all the time and across the whole globe. There are three types of airglow: dayglow, twilightglow and nightglow. Each is the result of sunlight interacting with the molecules in our atmosphere, but they have their own special way of forming.
Dayglow forms when sunlight strikes the daytime atmosphere. Some of the sunlight is absorbed by the molecules in the atmosphere, which gives them excess energy. They become excited. The molecules then release this energy as light, either at the same or slightly lower frequency (colour) as the light they absorbed. This light is much dimmer than daylight, so we can’t see it by eye.
Twilight glow is essentially the same as dayglow, but only the upper atmosphere is sunlit. The rest of the atmosphere and the observer on the ground are in darkness. So, unlike day glow, twilightglow is actually visible to us on the ground with the naked eye.
Chemiluminescence
The chemistry behind nightglow is different. There is no sunlight shining on the nighttime atmosphere. Instead, a process called “chemiluminescence” is responsible for the glowing atmosphere.
Sunlight deposits energy into the atmosphere during the day, some of which is transferred to oxygen molecules (e.g. O₂). This extra energy causes the oxygen molecules to rip apart into individual oxygen atoms. This happens particularly around 100km in altitude. However, atomic oxygen isn’t able to get rid of this excess energy easily and so acts as a “store” of energy for several hours.
Eventually the atomic oxygen does manage to “recombine”, once again forming molecular oxygen. The molecular oxygen then releases energy, again in the form of light. Several different colours are produced, including a “bright” green emission.

Airglow spotted in panoramic shot of the Very Large Telescope. Beletsky, CC BY-SA
In reality, the green nightglow isn’t particularly bright, it’s just the brightest of all nightglow emissions. Light pollution and cloudy skies will prevent sightings. If you’re lucky though, you might just be able to see it by eye or capture it on long-exposure photos.
Not to be confused with aurora
The green night glow emission is very similar to the famous green we see in the northern lights. This is unsurprising since it is produced by the same oxygen molecules as the green aurora. But the two phenomena are not related.
Aurora form when charged particles, such as electrons, bombard the Earth’s atmosphere. These charged particles, which started off at the sun and were accelerated in the Earth’s magnetosphere, collide with the atmospheric gases. They transfer energy, forcing the gases to emit light.

But it isn’t just the process behind them that is different. The aurora form in a ring around the magnetic poles (known as the auroral oval); whereas nightglow is emitted across the whole night sky. The aurora are very structured (due to the Earth’s magnetic field); whereas airglow is generally quite uniform. The extent of the aurora is affected by the strength of the solar wind; whereas airglow happens all the time.
Why then did we get a lot sightings from the UK recently, rather than all the time? The brightness of airglow correlates with the level of ultraviolet (UV) light being emitted from the sun – which varies over time. The time of year also seems to have an impact on the strength of airglow.

To maximise your chances of spotting airglow, you’ll want to take a long-exposure photograph of a clear, dark, night sky. Airglow can be spotted in any direction that is free of light pollution, at about 10⁰-20⁰ above the horizon.
Source: Beautiful green ‘airglow’ spotted by aurora hunters – but what is it?
Emerald green, fainter than the zodiacal light and visible on dark nights everywhere on Earth, airglow pervades the night sky from equator to pole. Airglow turns up in our time exposure photographs of the night sky as ghostly ripples of aurora-like light about 10-15 degrees above the horizon. Its similarity to the aurora is no coincidence. Both form at around the same altitude of 60-65 miles (100 km) and involve excitation of atoms and molecules, in particular oxygen. But different mechanisms tease them to glow.

Auroras get their spark from high-speed electrons and protons in the solar wind that bombard oxygen and nitrogen atoms and molecules. As excited electrons within those atoms return to their rest states, they emit photons of green and red light that create shimmering, colorful curtains of northern lights.
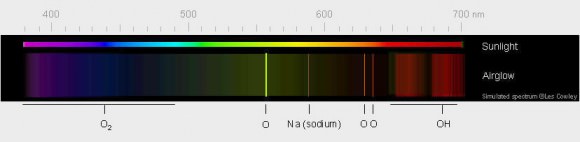
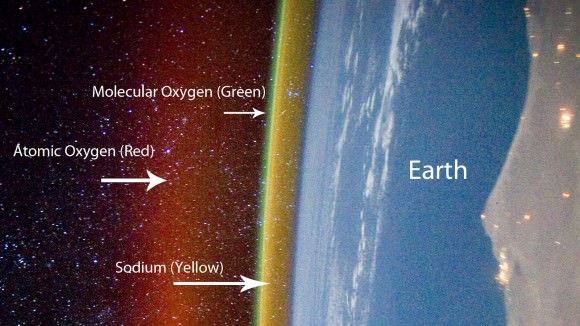
Airglow’s subtle radiance arises from excitation of a different kind. Ultraviolet light from the daytime sun ionizes or knocks electrons off of oxygen and nitrogen atoms and molecules; at night the electrons recombine with their host atoms, releasing energy as light of different colors including green, red, yellow and blue. The brightest emission, the one responsible for creating the green streaks and bands visible from the ground and orbit, stems from excited oxygen atoms beaming light at 557.7 nanometers, smack in the middle of the yellow-green parcel of spectrum where our eyes are most sensitive.


That’s not saying airglow is easy to see! For years I suspected streaks of what I thought were high clouds from my dark sky observing site even when maps and forecasts indicated pristine skies. Photography finally taught me to trust my eyes. I started noticing green streaks near the horizon in long-exposure astrophotos. At first I brushed it off as camera noise. Then I noticed how the ghostly stuff would slowly shape-shift over minutes and hours and from night to night. Gravity waves created by jet stream shear, wind flowing over mountain ranges and even thunderstorms in the lower atmosphere propagate up to the thermosphere to fashion airglow’s ever-changing contours.

Last month, on a particularly dark night, I made a dedicated sweep of the sky after my eyes had fully adapted to the darkness. A large swath of airglow spread south of the Big and Little Dipper. To the east, Pegasus and Andromeda harbored hazy spots of varying intensity, while brilliant Mars beamed through a long smear in Virgo.
To prove what I saw was real, I made the photos you see in this article and found they exactly matched my visual sightings. Except for color. Airglow is typically too faint to fire up the cone cells in our retinas responsible for color vision. The vague streaks and patches were best seen by moving your head around to pick out the contrast between them and the darker, airglow-free sky. No matter what part of the sky I looked, airglow poked its tenuous head. Indeed, if you were to travel anywhere on Earth, airglow would be your constant companion on dark nights, unlike the aurora which keeps to the polar regions. Warning – once you start seeing it, you
Airglow comes in different colors – let’s take a closer look at what causes them:
* Red – I’ve never seen it, but long-exposure photos often reveal red/pink mingled with the more common green. Excited oxygen atoms much higher up at 90-185 miles (150-300 km) radiating light at a different energy state are responsible. Excited -OH (hydroxyl) radicals give off deep red light in a process called chemoluminescence when they react with oxygen and nitrogen. Another chemoluminescent reaction takes place when oxygen and nitrogen molecules are busted apart by ultraviolet light high in the atmosphere and recombine to form nitric oxide (NO).
* Yellow – From sodium atoms around 57 miles (92 km) high. Sodium arrives from the breakup and vaporization of minerals in meteoroids as they burn up in the atmosphere as meteors.
* Blue – Weak emission from excited oxygen molecules approximately 59 miles (95 km) high.

Airglow varies time of day and night and season, reaching peak brightness about 10 degrees, where our line of sight passes through more air compared to the zenith where the light reaches minimum brightness. Since airglow is brightest around the time of solar maximum (about now), now is an ideal time to watch for it. Even cosmic rays striking molecules in the upper atmosphere make a contribution.
https://www.youtube.com/embed/zymQQP4B21Q
See lots of airglow and aurora from orbit in this video made using images taken from the space station.
If you removed the stars, the band of the Milky Way and the zodiacal light, airglow would still provide enough illumination to see your hand in front of your face at night. Through recombination and chemoluminescence, atoms and molecules creates an astounding array of colored light phenomena. We can’t escape the sun even on the darkest of nights.
Source: How to See Airglow, the Green Sheen of Night
In 2018, a new aurora-like discovery struck the world. From 2015 to 2016, citizen scientists reported 30 instances of a purple ribbon in the sky, with a green picket fence structure underneath. Now named STEVE, or Strong Thermal Emission Velocity Enhancement, this phenomenon is still new to scientists, who are working to understand all its details. What they do know is that STEVE is not a normal aurora—some think maybe it’s not an aurora at all—and a new finding about the formation of streaks within the structure brings scientists one step closer to solving the mystery.
“Often in physics, we build our understanding then test the extreme cases or test the cases in a different environment,” Elizabeth MacDonald, a space scientist at NASA’s Goddard Space Flight Center in Greenbelt, Maryland, explains. “STEVE is different than the usual aurora, but it is made of light and it is driven by the auroral system. In finding these tiny little streaks, we may be learning something fundamentally new in how green auroral light can be produced.”
These “tiny little streaks” are extraordinarily small point-like features within the green picket fence of STEVE. In a new paper for AGU Advances, researchers share their latest findings on these points. They suggest the streaks could be moving points of light—elongated in the images due to blur from the cameras. The tip of the streak in one image will line up with the end of the tail in the next image, contributing to this speculation from the scientists. However, there are still a lot of questions to be answered—determining whether the green light is a point or indeed a line, is one extra clue to help scientists figure out what causes green light.
“I’m not entirely sure about anything with respect to this phenomenon just yet,” Joshua Semeter, a professor at Boston University and first author on the paper, said. “You have other sequences where it looks like there is a tube-shaped structure that persists from image to image and doesn’t seem to conform to a moving point source, so we’re not really sure about that yet.”
STEVE as a whole is something that scientists are still working to label. Scientists tend to classify optical features in the sky into two categories: airglow and aurora. When airglow occurs at night, atoms in the atmosphere recombine and release some of their stored energy in the form of light, creating bright swaths of color. By studying the patterns in airglow, scientists can learn more about that area of the atmosphere, the ionosphere. To be classified as an aurora, on the other hand, that release of light must be caused by electron bombardment. These features are formed differently but also look different—airglow can occur across Earth, while auroras form in a broad ring around Earth’s magnetic poles.
“STEVE in general appears to not conform well to either one of those categories,” Semeter said. “The emissions are coming from mechanisms that we don’t fully understand just yet.”
STEVE’s purple emissions are likely a result of ions moving at a supersonic speed. The green emissions seem to be related to eddies, like the ones you might see forming in a river, moving more slowly than the other water around it. The green features are also moving more slowly than the structures in the purple emissions, and scientists speculate they could be caused by turbulence in the space particles—a brew of charged particles and magnetic field, called plasma—at these altitudes.
“We know this kind of turbulence occurs. There are people who base their entire careers on studying turbulence in the ionospheric plasma formed by very rapid flows.” Semeter said. “The evidence generally comes from radar measurements. We don’t ever have an optical signature.” Semeter suggests that when it comes to the appearance of STEVE, the flows in these instances are so extreme, that we can actually see them in the atmosphere. Two different angles of distinctive green streaks below a STEVE event on Aug. 31, 2016, near Carstairs, Alberta, Canada. Recent research about the formation of these streaks is allowing scientists to learn more about this aurora-like phenomenon. Credit: Copyright Neil Zeller, used with permission
“This paper is the tip of the iceberg in this new area of these tiny little pieces of the picket fence. Something we do in physics is try to chip away to increase our understanding,” MacDonald said. “This paper establishes the altitude range and some of the techniques we can use to identify these features, then they can be better resolved in other observations.”
To establish the altitude range and identify these features, the scientists extensively used photos and videos captured by citizen scientists.
“Citizen scientists are the ones who brought the STEVE phenomenon to the scientists’ attention. Their photos are typically longer time lapse than our traditional scientific observations,” MacDonald said. “Citizen scientists don’t get into the patterns that scientists get into. They do things differently. They are free to move the camera around and take whatever exposure they want.” However, to make this new discovery of the points within STEVE, photographers actually took shorter exposure photographs to capture this movement.
To get those photographs, citizen scientists spend hours in the freezing cold, late at night, waiting for an aurora—or hopefully STEVE—to appear. While data can indicate if an aurora will show up, indicators for STEVE haven’t been identified yet. However, the aurora chasers show up and take pictures anyway.
[…]
Source: Aurora-chasing citizen scientists help discover a new feature of STEVE

