2000 m above ground level (AGL), winds are stronger and much, much more consistent than they are at surface. Even if the Earth were a perfect sphere, there’d be a sluggish boundry layer at the surface, but since it’s got all these interesting bumps and bits and bobs, it’s not just sluggish but horribly turbulent, too. Getting above that, as much as possible, is why wind turbines are on big towers. Rather than build really big tower, Beijing Lanyi Yunchuan Energy Technology Co. has gone for a more ambitious approach: an aerostat to take power from the steady winds found at high altitude. Ambitiously called the Stratosphere Airborne Wind Energy System (SAWES), the megawatt-scale prototype has recently begun feeding into the grid in Yibin, Sichuan Province.
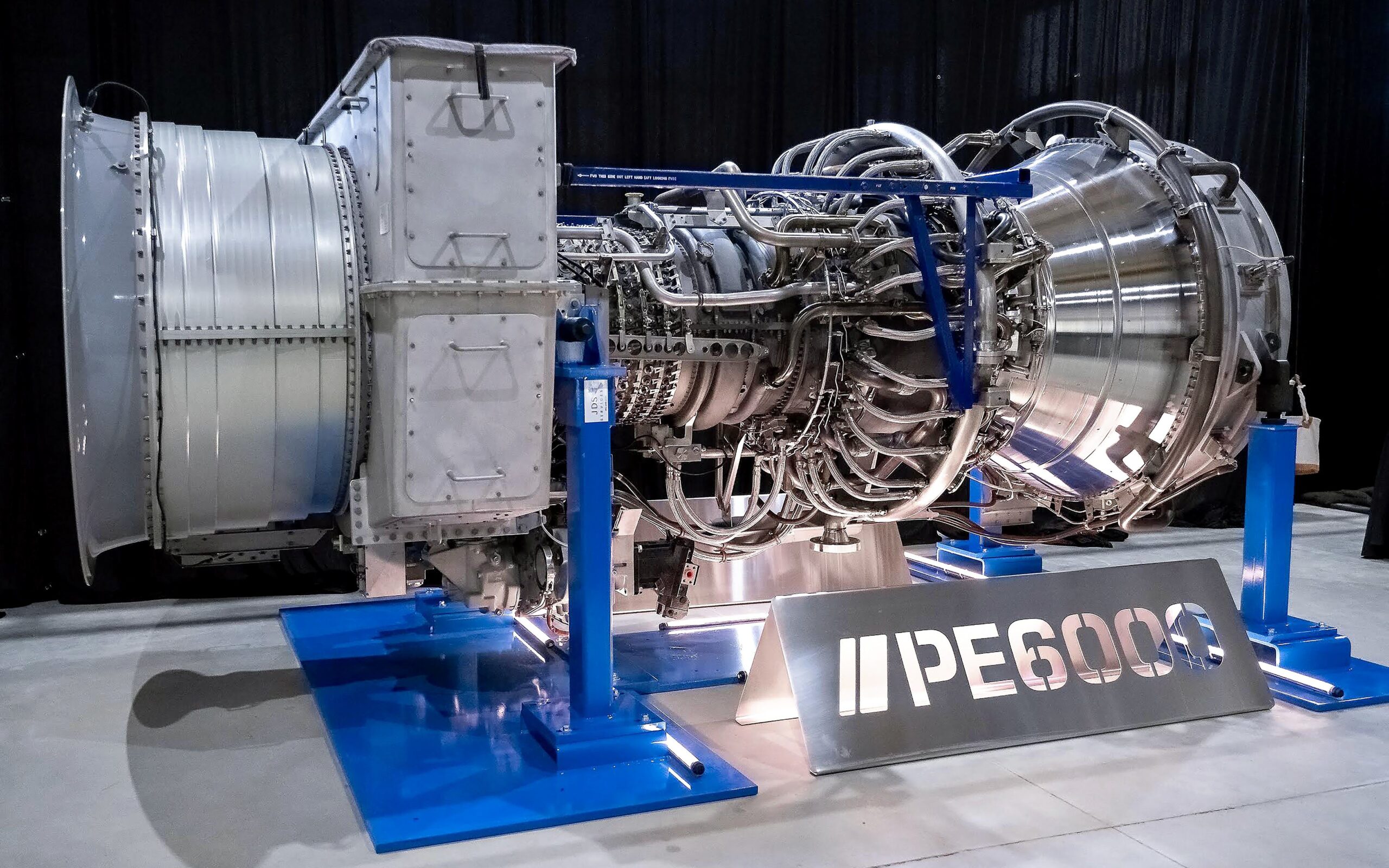
The name might be a bit ambitious, since its 2000 m test flight is only one tenth of the way to the stratosphere, but Yibin isn’t a bad choice for testing: as it is well inland, the S2000 prototype won’t have to contend with typhoons or other ocean storms. The prototype is arguably as ambitious as the name: its 12 flying turbines have a peak capacity of three megawatts. True, there are larger turbines in wind farms right now, but at 60 m in length and 40 m in diameter, the S2000 has a lot of room to grow before hitting any kind of limit or even record for aerostats. We’re particularly interested in the double-hull construction– it would seem the ring of the outer gas bag would do a good job funneling and accelerating air into those turbines, but we’d love to see some wind tunnel testing or even CFD renderings of what’s going on in there.

During its first test flight in January 2026, the system generated generated 385 kilowatt-hours of electricity over the course of 30 minutes. That means it averaged about 25% capacity for the test, which is a good safe start. Doubtless the engineers have a full suite of test flights planned to demonstrate the endurance and power production capabilities of this prototype. Longer flights at higher capacity may have already happened by the time you read this.
Flying wind turbines isn’t a new idea by any means; a few years ago we featured this homemade kite generator, and the pros have been in on it too. Using helium instead represents an interesting design choice–on the plus side, its probably easier to control, and obviously allowing large structures, but the downside is the added cost of the gas. It will be interesting to see how it develops.
We’re willing to bet it catches on faster than harvesting wind energy from trees.
All images from Beijing Lanyi Yunchuan Energy Technology Co., Ltd.
Source: Wind Power Is Taking Off In China– All The Way To 2000 M AGL | Hackaday