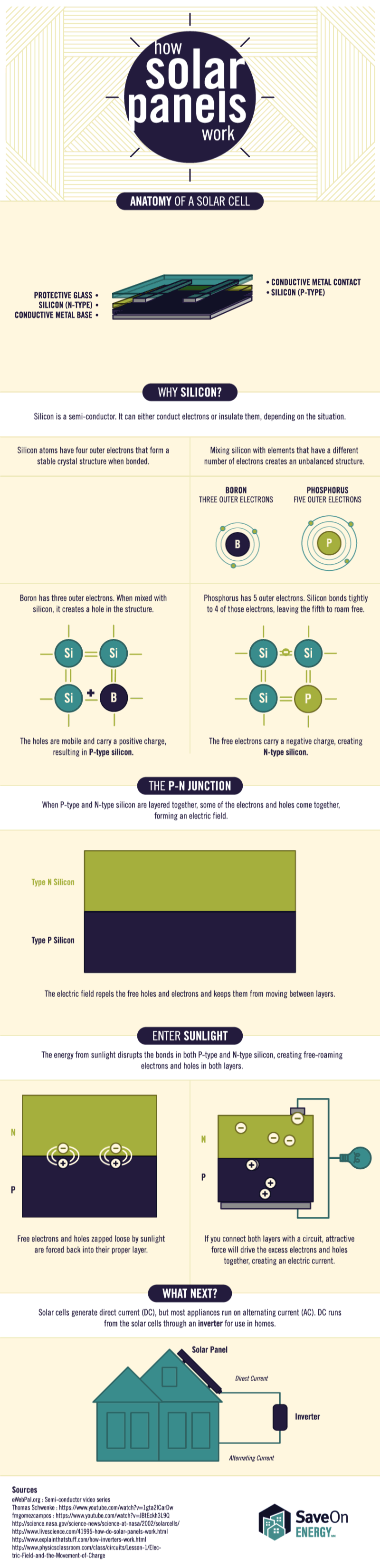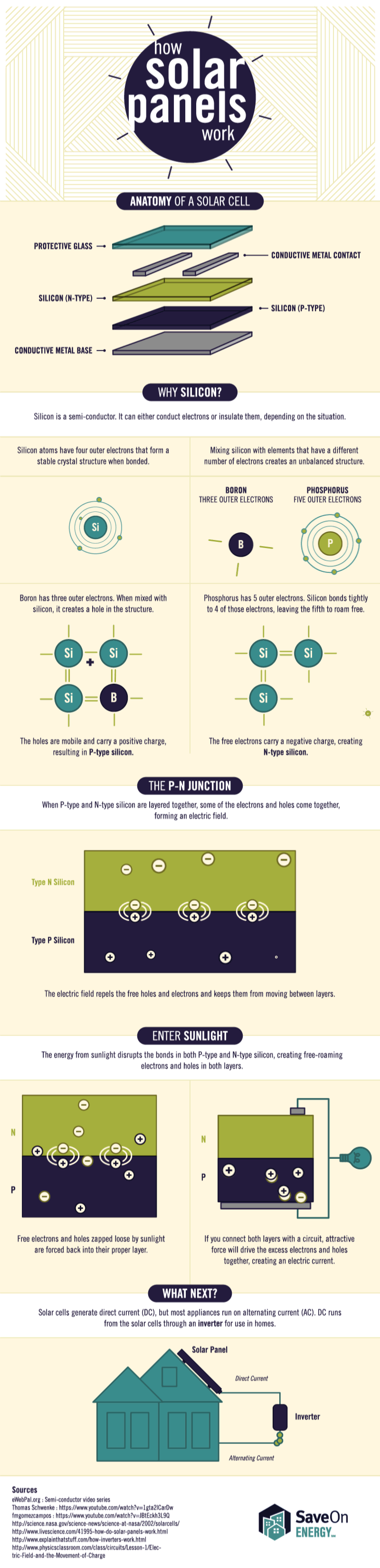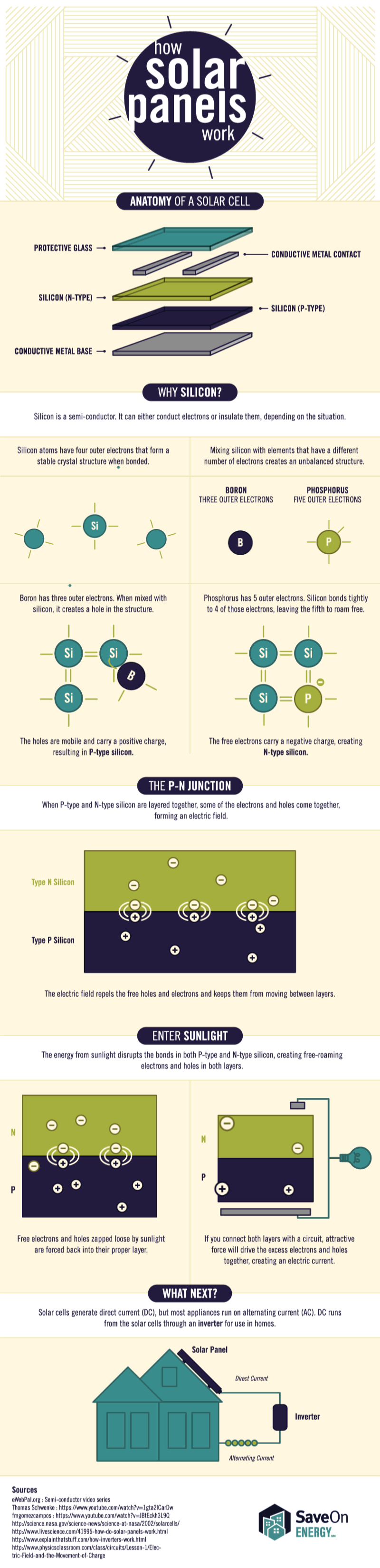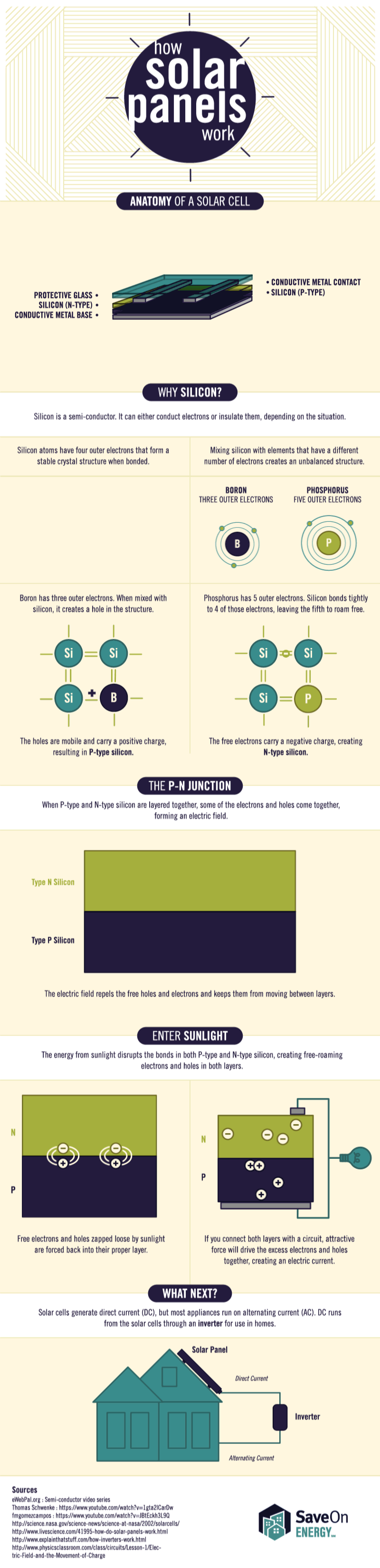
Tesla’s Big Battery, located in southern Australia, just got hit with a federal lawsuit for failing to provide the crucial grid support it once promised it could.
Built by Tesla in 2017, the 150-megawatt battery supplies 189 megawatt-hours of storage and was designed to support the grid when it becomes overloaded. Now operated by French renewable energy producer Neoen, it supplies storage for the adjacent Hornsdale wind farm, using clean energy to fill gaps that coal power leaves behind. It made waves at the time of its construction for being the largest lithium-ion battery in the world—though it’s now been superseded by another Tesla battery, the 300-megawatt Victorian Big Battery, also in Australia, which caught fire in July.
On Wednesday, the Australian Energy Regulator (AER), the body that oversees the country’s wholesale electricity and gas markets, announced it had filed a federal lawsuit against the Hornsdale Power Reserve (HPR)—the energy storage system that owns the Tesla battery—for failing to provide “frequency control ancillary services” numerous times over the course of four months in the summer and fall of 2019. In other words, the battery was supposed to supply grid backup when a primary power source, like a coal plant, fails.
The HPR’s alleged pattern of failures was first brought to light during a disruption to a nearby coal plant in 2019, according to the regulator. When the nearby Queensland’s Kogan Creek power station tripped on October 9, 2019, the HPR was called on to offer grid backup, having made offers to the Australian Energy Market Operator (AEMO) to do so.
But the power reserve failed to provide the level of grid support that AEMO expected, and, in fact, was never able to do so in the first place, the lawsuit alleges, despite making money off of offering them. Though HPR did step in eventually, and no outages were recorded, the incident spurred investigation into a number of similar failures over the course of July to November 2019. The reserve’s failure to support the grid in the way it promised created “a risk to power system security and stability,” a press release on the lawsuit says.
“Contingency FCAS providers receive payment from AEMO to be on standby to provide the services they offer,” Clare Savage, chair of AER, said in a press release on the suit. “We expect providers to be in a position, and remain in a position, to respond when called upon by AEMO.”
[…]
Source: A Tesla Big Battery Is Getting Sued Over Power Grid Failures In Australia